Are you asking for 'qbd thesis'? You will find questions and answers on the subject here.
Table of contents
- Qbd thesis in 2021
- Qbd thesis 02
- Qbd thesis 03
- Qbd thesis 04
- Qbd thesis 05
- Qbd thesis 06
- Qbd thesis 07
- Qbd thesis 08
Qbd thesis in 2021
 This image demonstrates qbd thesis.
This image demonstrates qbd thesis.
Qbd thesis 02
 This image representes Qbd thesis 02.
This image representes Qbd thesis 02.
Qbd thesis 03
 This image shows Qbd thesis 03.
This image shows Qbd thesis 03.
Qbd thesis 04
 This picture shows Qbd thesis 04.
This picture shows Qbd thesis 04.
Qbd thesis 05
 This picture demonstrates Qbd thesis 05.
This picture demonstrates Qbd thesis 05.
Qbd thesis 06
 This picture representes Qbd thesis 06.
This picture representes Qbd thesis 06.
Qbd thesis 07
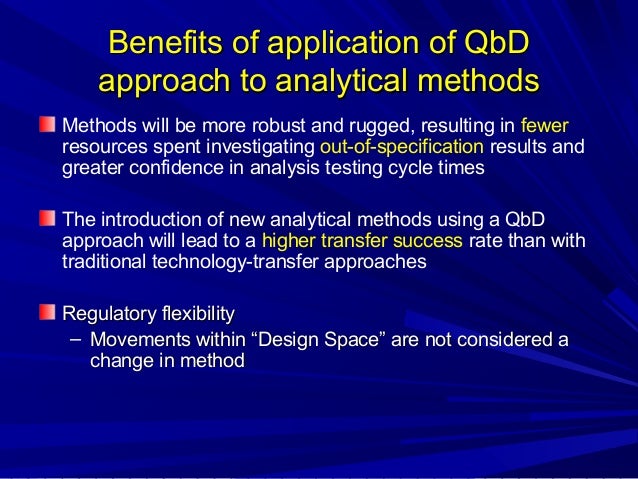 This picture representes Qbd thesis 07.
This picture representes Qbd thesis 07.
Qbd thesis 08
 This picture representes Qbd thesis 08.
This picture representes Qbd thesis 08.
Who is the master's thesis advisor for Near East University?
MASTERS THESIS PHARMACEUTICAL TECHNOLOGY DEPARTMENT ADVISOR Assoc. Prof. Dr. YILDIZ ÖZALP 2019-NICOSIA Approval Thesis submitted to the Institute of Health Sciences of Near East University in partial fulfillment of the requirements for the degree of Master of Science in Pharmaceutical Technology.
What does QBD stand for in regulatory guidance?
QbD is “Woven” into Regulatory Guidance Documents ICH • Primarily ICH Q8 through Q11 • Q8- Pharmaceutical Development • Q9- Quality Risk Management • Q10- Pharmaceutical Quality System • Q11- Development and Manufacture of Drug Substances
How is quality by Design ( QbD ) approach used?
Quality by Design (QbD) approach was used to facilitate method development. EBZ was exposed to different stress conditions, including hydrolytic (acid, base, neutral), oxidative, thermal and photolytic.
Who are the members of the thesis committee?
According to the relevant articles of the Near East University Postgraduate Study – Education and Examination Regulations, this thesis has been approved by the members of the Thesis Committee and the decision of the Board of Directors of the Institute. STATEMENT (DECLARATION)
Last Update: Oct 2021